Atomic Structure
Every Atom Needs Some Structure
Before we can learn about all things atom, we should probably start with the most basic question: what is an atom?An atom is a unit of matter and is the most basic unit of an element. It cannot be broken by any chemical processes.
Remember our good friend John Dalton? He imagined an atom was both extremely small and indivisible. This was a good starting point, but it doesn't tell the whole story. In the 1850s, a series of experiments were completed that demonstrated that atoms actually possess internal structure and are made up of even smaller particles called subatomic particles. The three most basic subatomic particles are electrons, protons, and neutrons.
We'd like to introduce you to another famous scientist, Ernest Rutherford. He was a New Zealand-born scientist and is considered to be one of the great experimentalists of all time. If there was a science hall of fame, he'd totally be in it. He had a way of figuring things out. He is known as the father of nuclear physics and earned a Nobel Prize in Chemistry in 1908 for his outstanding work.1

Ernest Rutherford 1871-1937. (Image from here.)
So what exactly did this guy do to look so smug in that photo? And what does it have to do with the atom? Rutherford was the scientist who discovered that atoms have a nucleus (a center) containing protons. A few years later, other scientists discovered that the nucleus is also home to the neutron and electron.
The nucleus is incredibly small. We mean really small. It's also very dense when compared to the rest of the atom. Check out these dimensions: Atoms have diameters that measure around 10-10 meters, while the diameter of a nucleus is typically around 10-15 meters. If the entire atom were scaled up to the size of a baseball stadium, the nucleus would be about the size of a peanut.
Let's get our learn on and focus on subatomic specifics.
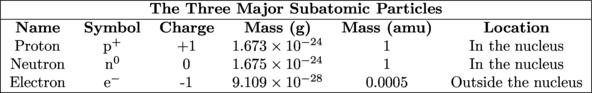
Subatomic Particles – Electrons, Protons, and Neutrons
Let's start with the proton—that loveable positively charged subatomic particle. These guys are located inside the nucleus. How are all of these positively charged particles crammed together inside a little space if like charges repel each other? It's the Force, young Jedi. Forces within the nucleus counteract this proton-proton repulsion and hold the nucleus together. Physicists call these forces nuclear glue.
We know that the nucleus is super small compared to the rest of the atom, so it might be surprising to hear that it actually contains most of the mass of the atom. For all practical purposes, the mass of the entire atom is pretty much the mass of the nucleus. Crazy, huh?
Atoms are neutral in charge. (Yes atoms can be positively or negatively charged as well, but we'll cover that later). To balance out the positively charged protons we need a negatively charged subatomic particle: the electron. Chemical reactivity, chemical bonding, the shape of molecules, and even electricity are based on where the electrons in an atom are located. So far we've only mentioned that the electrons are not located in the nucleus. Where are the electrons in an atom located?
The earliest models of the atom had the electrons buzzing around the nucleus randomly. As scientists completed more experiments and became atomic experts they realized that this representation probably wasn't right. Today, scientists use a highly mathematical model called the quantum mechanical model to locate electrons and better represent the structure of the atom. For now, just be glad they figured this out and you didn't have to.
This complicated model is based on quantum theory, which says that matter also has properties associated with waves. In other words, electrons are particles of matter with a particular mass but they also behave as if they were a wave. In fact all matter (including you, me, and Lady Gaga) possess particle properties and wave properties. The smaller a piece of matter is the more wave-like and less particle-like it is. You and are huge pieces of matter so our wave-like properties are practically non-existent. Just remember that quantum theory and waves go hand-in-hand.
How does this help us determine an electron's location? According to quantum theory, it's impossible to know an electron's exact position and momentum at the same time. This is known as the uncertainty principle. In other words, if you know the exact location of an electron you have zero information about its momentum (and remember electrons are in constant motion), or if you know its exact momentum you know nothing about its location. The next time your mom inquires about your location, try telling her you are currently in your car going exactly 40 mph, therefore you have zero idea where you are. That's the uncertainty principle.
At this point we're still uncertain about an electron's location. Instead of being obsessed with determining the exact location of any given electron at any specific time, scientists decided it was a-okay to just figure out the probability of an electron's location at any given time. In other words, they replaced certainty with probability. They know there's a really good chance the electron is somewhere in a calculated volume around the atom. From all this probability stuff came the idea of orbitals (or electron clouds).
Orbitals are volumes of space in which an electron is most likely to be found. In later sections you'll learn all about orbitals including their shapes, energies, and all that other good stuff. For now, just know that electrons are negatively charged subatomic particles located outside the nucleus in orbitals.

Orbitals come in many shapes and sizes.
We have one subatomic particle left to discuss: the neutron. Neutrons are the next-door neighbors of protons. They also reside in the dense and compact nucleus. They are electrically neutral particles with a mass slightly greater than that of protons. Not all elements of the same atom have the same number of neutrons, but we're getting a little ahead of ourselves. Tune into the next section to learn all about neutrons and how they relate to elemental isotopes.