Geometry of Molecules
This Guide is Shaping Up
Congratulations, you're a fortune teller. Maybe you're not exactly a lady in a small room with a crystal ball, but you can make accurate predictions when it comes to molecules. You can predict what shape a particular molecule will have. This might not sound like a great party trick, but it will be useful for the next chemistry test. Excited to ace this geometry stuff? That's what we thought.The first thing we need to know is when electrons get too close to each other they begin to repel. Just like people, electrons need their own space sometimes. When atoms come together to form a molecule, the molecule will take the shape that keeps its different electron pairs as far away from each other as possible, in order to minimize these repulsive forces.
This is known as the VSEPR theory (Valence Shell Electron-Pair Repulsion). We will use this theory to help predict the shapes and three-dimensional geometries that molecules will form.
The basic shapes we're going to learn about are:
1. Linear
2. Bent
3. Trigonal planar
4. Tetrahedral
5. Trigonal pyramidal
There are more shapes within each of these basic shapes, but we won't trouble you with all of the terminology right now.
Check out some of the types of molecular geometry that VSEPR can predict:
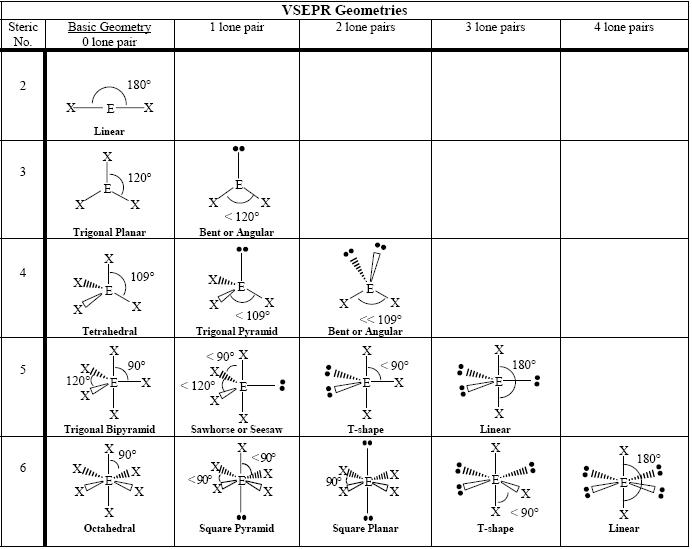
The number of electron pairs around the central atom determines the basic shape of the molecule. The very first and absolutely essential step is to first draw a Lewis dot structure of the molecule in question.
Focus all of your attention on the central atom. How many "things" does it have coming off of it? By "things," we mean single bonds, double bonds, triple bonds, or lone pairs. It's important not to count a double bond as two things. In our method, a double bond is just one thing.
If the central atom only has two things coming off of it, the molecule is linear. The chart above uses the example of CO2. The central carbon atom has two things coming off of it in the form of two double bonds. The central atom determines the molecular geometry, so the molecule is linear.
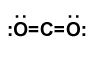
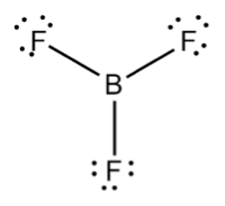
What about the BF3 molecule? Below, we've drawn a Lewis dot structure with three things coming off of the central B atom. This type of geometry is called trigonal planar.
What if there are three things connected to the central atom, but one of those things is a lone pair? If we draw out the Lewis dot structure for NO2- we'll observe such a phenomenon. In these cases we call the structure bent, because one of the things does not connect to another atom.
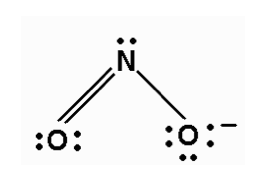
Why do we care about the existence of lone pairs? Electrons repel each other when they get too close. For that reason, lone pairs and bonded electrons maximize the distance between themselves according to specific geometries. Picture a bent NO2- molecule. Can we draw the molecule in another way to maximize the distance between the single bonds and the lone electron pair connected to the central atom? Nope.
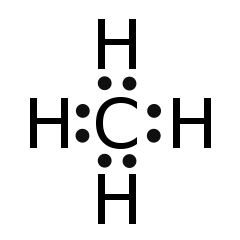
Let's move on. Draw out a Lewis dot structure for CH4, our favorite gas. If drawn correctly, the central carbon atom has four "things" coming off of it. To be more specific, it has a single bond to each of the four hydrogen atoms. To maximize the distance between all four bonds, the molecule arranges its "things" into a tetrahedral geometry with 109.5o between each leg.
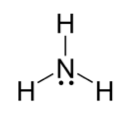
What if one of the things is not a covalent bond but a lone pair? Draw out the Lewis dot for NH3. You'll draw a central N atom with three H atoms and one lone pair. The repulsion maximizes the distance between each thing and we get a tetrahedral shape. However, one of the things is a lone pair so we call this geometry trigonal pyramidal. Easy peasy.

Here's a scenario: A couple of hydrogen atoms and an oxygen atom walk into your Parlour of Fortune. You sit them down behind your crystal ball. They want to know what shape they would take on if they all bonded. You immediately turn on your special effects to "wow" them—you know, your basic pink smoke, twinkling stars, and the floating crystal ball that you rigged up with magnets. You want to keep up appearances, and image is everything.
Based on the tools you were given, you look to the central atom; in this case, that would be the oxygen atom. You look at the number of electron pairs around the oxygen atom, and you notice 4 electron pairs. According to your astute calculations this means that it has sp3 hybridization and its basic shape will be tetrahedral. Your clients are very impressed, but you've got more to tell them.
After the bonds have been made with the two hydrogen atoms you notice there are two lone pairs of electrons remaining around the oxygen atom. This means that their geometry is actually bent. Your clients are very impressed. They immediately bond and become a puddle of water on your floor. Better go get a towel.