ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Laws of Thermodynamics Videos 15 videos
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?
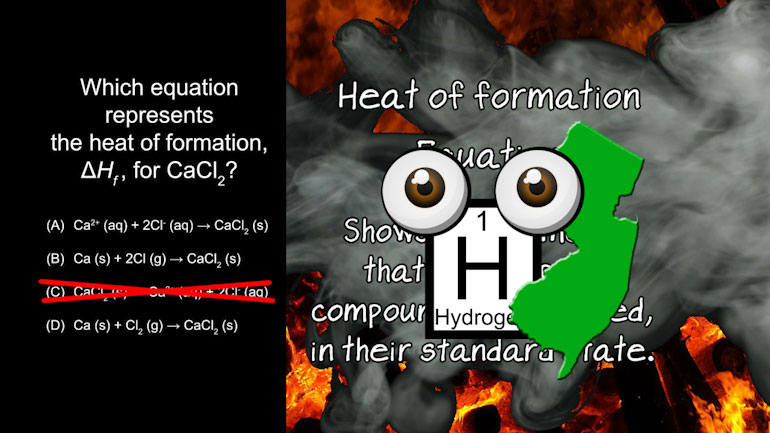
AP® Chemistry: Laws of Thermodynamics Drill 1, Problem 1. Which equation represents the heat of formation for Calcium Chloride?
AP Chemistry 1.1 Laws of Thermodynamics 228 Views
Share It!
Description:
AP® Chemistry: Laws of Thermodynamics Drill 1, Problem 1. Which equation represents the heat of formation for Calcium Chloride?
Transcript
- 00:03
Here's your shmoop du jour, brought to you by Calcium Chloride. Roads need it to grow
- 00:08
up big and strong. Or... to keep from getting icy, or whatever.
- 00:13
Which equation represents the heat of formation for Calcium Chloride?
- 00:18
And here are your potential answers...
- 00:26
Even if we have no idea about what the heat of formation is... let's think about it. FORMATION.
Full Transcript
- 00:32
We probably want the compound calcium chloride to be on the RIGHT side of the equation...
- 00:36
since that's what's being FORMED. Which means we can immediately eliminate C.
- 00:44
So now to learn what a heat of formation equation...is.
- 00:49
A heat of formation equation shows the elements that make up the compound to be formed, in
- 00:53
their standard state. It's important that they're in their standard
- 00:57
state...which is basically whether they're solid, liquid, or gas at room temperature.
- 01:02
Calcium is a solid at room temperature, and chlorine is a gas at room temperature...
- 01:06
but a diatomic molecule...
- 01:13
Meaning that it looks like Cl 2, not 2 Cl.
- 01:17
These two molecules are in their standard states in option (D).
- 01:21
D's our answer. We haven't had so much fun with formation
- 01:24
since we got that lifetime supply of Play-doh...
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?