ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Structure and Arrangement of Atoms Videos 9 videos
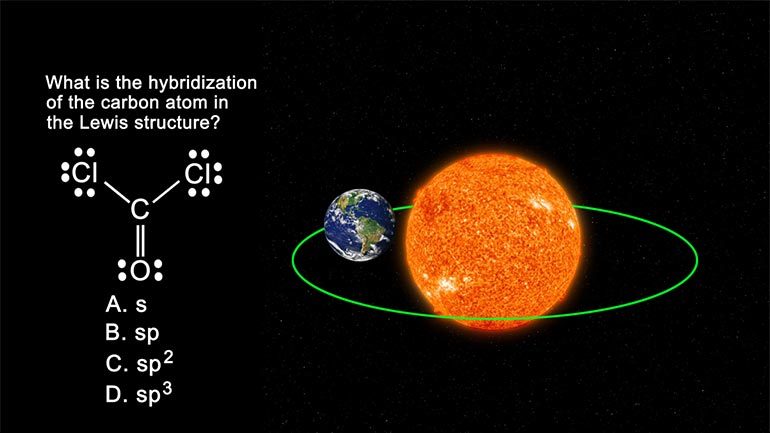
AP Chemistry: Structure of Atoms Drill 1, Problem 5. What is the hybridization of the carbon atom in the Lewis structure?
AP Chemistry 2.5 Structure and Arrangement of Atoms. Under which of the following conditions would a real gas behave more like an ideal gas?
AP Chemistry 3.5 Structure and Arrangement of Atoms. Which of the following is true regarding solids?
AP Chemistry 1.5 Structure and Arrangement of Atoms 306 Views
Share It!
Description:
AP Chemistry: Structure of Atoms Drill 1, Problem 5. What is the hybridization of the carbon atom in the Lewis structure?
Transcript
- 00:03
Here's your shmoop du jour, brought to you by Carbon Atoms. By far the easiest atoms to copy.
- 00:13
What is the hybridization of the carbon atom in the Lewis structure?
- 00:19
And here are your potential answers...
- 00:26
OK, so the key to answering this question is to understand what hybridization is.
- 00:32
You've probably heard of hybrid cars...or even dog hybrids, like a labradoodle.
Full Transcript
- 00:37
They're super energy-efficient.
- 00:40
The concept of hybridization derives from mixing the
- 00:43
originals of something into a new mix, or hybrid.
- 00:47
In chemistry, hybridization refers to the mixing of atomic orbitals.
- 00:56
Just as there's a path we can follow to define the orbit of a planet around the sun, there's
- 01:01
also a path we can follow to define the way an electron orbits the nucleus of an atom...also
- 01:06
called an atomic orbital.
- 01:08
Hybridization in chemistry refers to the mixing of atomic orbitals into hybrid orbitals which
- 01:14
allow electrons to form chemical bonds.
- 01:17
In fact, if atoms didn't "hybridize", some of these chemical bonds couldn't form because
- 01:21
they wouldn't have the right shape or structure.
- 01:24
Here, hybridization allows the Carbon to single bond to two chlorine atoms and double bond
- 01:30
to the single oxygen atom. Oooh... edgy.
- 01:34
Now onto the REAL question. How do we figure out what the hybridization of the carbon atom is?
- 01:40
When determining hybridization, we can just count the number of other atoms that the atom
- 01:44
in question is connected to.
- 01:46
Carbon is connected to two chlorine atoms, and one oxygen atom.
- 01:51
It doesn't matter that carbon is double-bound to oxygen; it is still only bound to three atoms.
- 01:56
Three atoms means three hybridized orbitals.
- 02:00
But wait, our answer choices aren't just numbers... in chemistry notation, we can add the exponents
- 02:07
in s and p to find the number of hybridized orbitals it represents.
- 02:12
Looking at answer C, we have the exponent in s...1 plus the exponent in p... 2. 1 plus
- 02:19
2 is... give us a second... oh, right. 3.
- 02:22
So our answer is C.
- 02:24
As in "carbonite."
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?