ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Probability and Systems Videos 8 videos
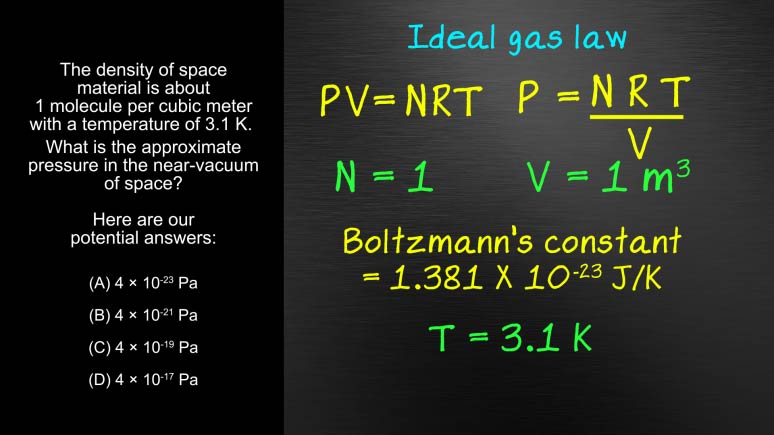
AP Physics 2: 1.3 Probability and Systems. What is the approximate pressure in the near-vacuum of space?
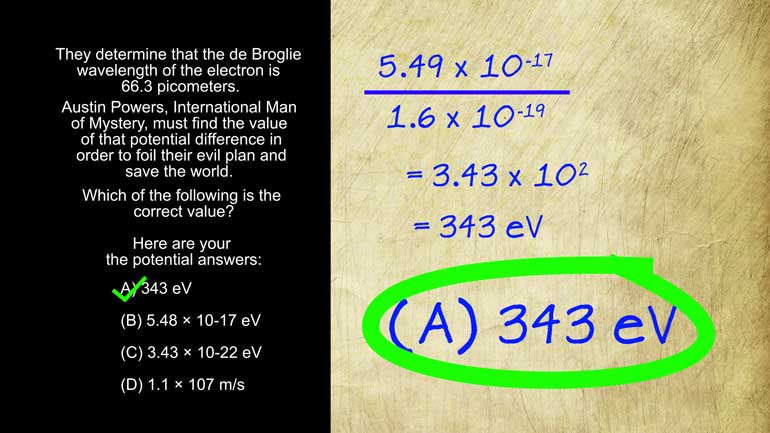
AP Physics 2: 2.2 Probability and Systems. What is the mass number of J?
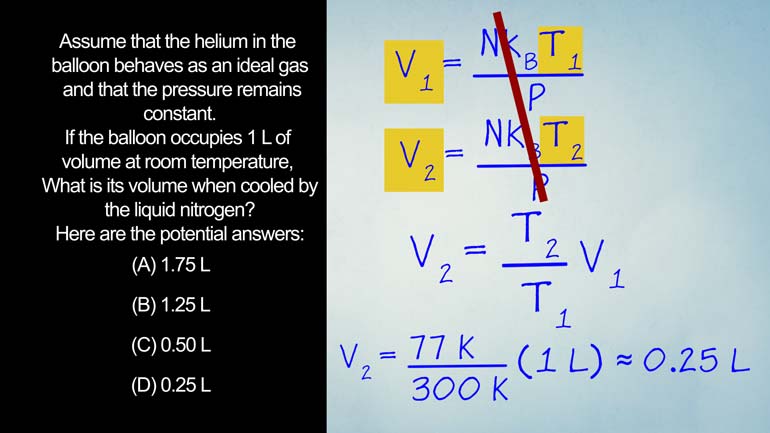
AP Physics 2: 1.2 Probability and Systems. What is the volume of the balloon when cooled with liquid nitrogen?
AP Physics 2: 1.2 Probability and Systems 172 Views
Share It!
Description:
AP Physics 2: 1.2 Probability and Systems. What is the volume of the balloon when cooled with liquid nitrogen?
Transcript
- 00:00
Thank you We sneak and here's your shmoop du jour
- 00:05
brought to you by liquid nitrogen which is great for
- 00:08
all sorts of physics thing Quit Well not so great
- 00:10
My swimming pool here's Your question assumed that the helium
- 00:15
in the balloon behaves is an ideal gas and the
Full Transcript
- 00:18
pressure remains constant that the balloon occupies one leader of
- 00:22
volume at room temperature what's its volume when cooled by
- 00:26
the liquid nitrogen and hear the financial answer I'm leaving
- 00:33
This experiment calls for the ideal gas law thiss law
- 00:36
explains how an ideal gas will act We assume that
- 00:39
being ideal in all it'll keep its room clean and
- 00:42
always be polite No wait That's not right And i
- 00:44
feel guest is a hypothetical gas that will always act
- 00:47
predictably Formula for an ideal gas law is pv equals
- 00:51
and katie this thing in this glacier p is pressure
- 00:55
be is volume and is the number of molecules Hey
- 00:58
sabi is bosman's constant and he is the temperature in
- 01:02
kelvin because it's the coolest Okay now that we have
- 01:07
that jan di door brains let's take another look at
- 01:09
our experiment Remember we're dropping a bullet gets a liquid
- 01:12
nitrogen because that's our idea of fun on a friday
- 01:15
night judges the balloon is sealed so the amount of
- 01:18
particles and pressure remain the same And remember we're trying
- 01:21
to figure out how the change in temperature effects the
- 01:23
change in volume Let's look at the two equations from
- 01:26
different volumes Since we're solving for volume we'll just rearrange
- 01:29
the equations by dividing both sides By p we could
- 01:33
dividing rearrange the equations canceling out everything but the volumes
- 01:36
And the temps visa two equals t's up to over
- 01:39
t someone times visa of one lycan in the Numbers
- 01:42
We have 77 degrees kelvin over three hundred reason calvin
- 01:45
times one leader which means the volume is about equal
- 01:49
to a point two five leaders and that makes me
- 01:51
the correct answer Well so there we have it Now
- 01:55
we can go fun playing around with liquid nitrogen and
- 01:58
just remember to wear gloves No one wants to end 00:02:00.643 --> [endTime] up with a hand sickle Well
Related Videos
AP Physics 2: 1.1 Properties of Objects and Systems. What is the magnitude and direction of the conventional current in this wire?
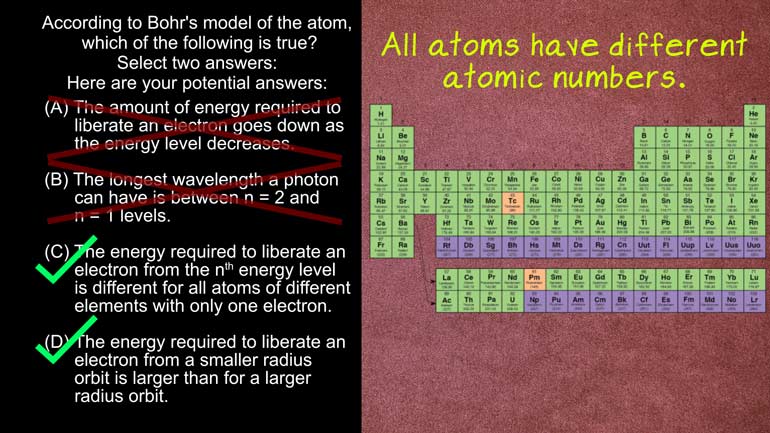
AP Physics 2: 1.5 Properties of Objects and Systems. According to the Bohr's model of the atom, which of the following are true?
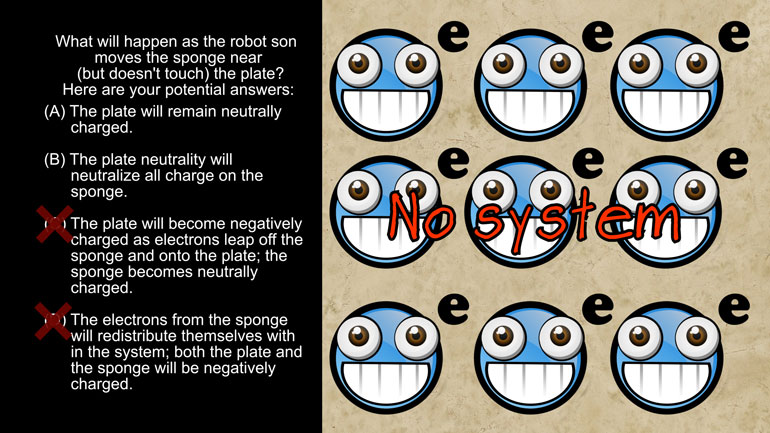
AP Physics 2: 2.2 Properties of Objects and Systems. What will happen as the robot son moves the sponge near (but doesn't touch) the plate?
AP Physics 2: 2.4 Properties of Objects and Systems. How could you show the carnival barker an emission spectrum?