ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
AP Chemistry Videos 45 videos
AP Chemistry 3.5 Chemical Reaction Rates. How much 85Kr will we need to collect?
Want to pull an Oliver Twist and ask us for more? We've gotcha covered. Head over to /video/subjects/test-prep/ap/ap-chemistry/ for more AP Ch...
AP Chemistry 1.3 Forming and Breaking Bonds. Which of the following ions are spectator ions?
AP Chem 1.3 Laws of Thermodynamics 17 Views
Share It!
Description:
AP Chem 1.3 Laws of Thermodynamics. How much energy would be required to change the temperature of 200 g of aluminum as described in the video?
Transcript
- 00:04
Here’s your Shmoop du jour, brought to you by aluminum. [Plane in the sky]
- 00:07
From airplane wings to surgical instruments, aluminum is useful in many ways…
- 00:12
…. but soda cans still take the cake for us. [Guy drinking soda on a plane]
- 00:15
Ah.
- 00:16
Refreshing. [Guy chucks the can away]
Full Transcript
- 00:17
Here’s our question:
- 00:18
Aluminum is a metal and a key raw material.
- 00:20
It is used to makes soda cans, foil, and even airplanes.
- 00:24
How much energy would be required to change the temperature of 200.0 g of aluminum from [The question being written out]
- 00:30
10.0 °C to 25.0 °C?
- 00:33
The specific heat of aluminum is 0.902 J/g °C.
- 00:39
And here are the potential answers, alright a bunch of joules [The answers appear]
- 00:44
Alright, lets see (glug, glug, glug) ahhh, seventh can today.
- 00:48
Okay, now that we’re all sufficiently hydrated, let’s get down to defeat the Huns… [Man has his fists up]
- 00:52
…uh. [Guy looks confused]
- 00:53
Sorry, we meant "to defeat this problem."
- 00:55
We're always getting chemistry and Mulan confused. [People in a chemsitry lab]
- 00:58
To solve today’s calorimetry question, we need to use this equation which relates [Teacher writing on a blackboard]
- 01:04
the amount of heat or energy transferred, Q, with a material’s mass, m, heat capacity,
- 01:11
C, and change in temperature, delta T. Phew.
- 01:14
That was a mouthful.
- 01:17
Quick, someone give us an eighth can of soda so we can rehydrate. [Guy drinking another can of soda with a pile of empties behind him]
- 01:19
Ah, much better.
- 01:20
So we’re trying to find Q, the amount of heat or energy transferred into the aluminum [Guy collapses onto the floor]
- 01:24
to heat it up.
- 01:25
From the problem statement, we know that the mass of aluminum that is heated is 200g
- 01:29
We also know that the specific heat is 0.902 joules per gram per degree
- 01:33
Celsius.
- 01:34
Finally, we know the aluminum is heated from 10 °C to 25 °C, which means a change [The equation working is written on the blackboard]
- 01:39
in temperature is 15 °C.
- 01:41
If we multiply this all out, we find that Q is 2,706 joules.
- 01:46
Rounding down to 2,700, this is answer choice (D).
- 01:50
What better way to celebrate our success than with a delicious can of soda? [Guy drinking soda on a plane again]
- 01:52
Ugh, that one was kind of sickening. …and why are our teeth suddenly falling
- 01:56
out? [Guy is crying]
- 01:57
Only one thing to do.
- 01:58
Relax, crack open another can. [Guy smiles and some of his teeth have fallen out]
Related Videos
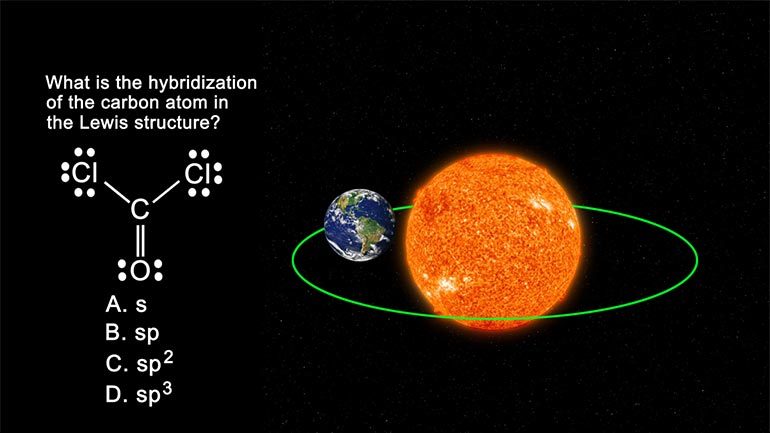
AP Chemistry: Structure of Atoms Drill 1, Problem 5. What is the hybridization of the carbon atom in the Lewis structure?
AP Chemistry DBQ/Free Response. Perform the following calculations.
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?