ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
AP Chemistry Videos 45 videos
AP Chemistry 3.5 Chemical Reaction Rates. How much 85Kr will we need to collect?
Want to pull an Oliver Twist and ask us for more? We've gotcha covered. Head over to /video/subjects/test-prep/ap/ap-chemistry/ for more AP Ch...
AP Chemistry 1.3 Forming and Breaking Bonds. Which of the following ions are spectator ions?
AP Chemistry 1.5 Laws of Thermodynamics 10 Views
Share It!
Description:
AP Chemistry 1.5 Laws of Thermodynamics. What is the heat of combustion of propene C 3 H 6 ?
Transcript
- 00:03
Here’s your Shmoop du jour, brought to you by oil refining.
- 00:07
Once it's finished being refined, we hear it has a lovely debutante ball. [Person walks down staircase in a gown]
- 00:12
Okay, here’s our question:
- 00:13
Propene is a byproduct of the oil refining process.
- 00:17
What is the heat of combustion of propene, C3H6, given the following information?
Full Transcript
- 00:22
We’re given several chemical reaction equations and reaction enthalpies.
- 00:30
And here are the potential answers: Okay, let's not be scared off by all those
- 00:37
letters and numbers.
- 00:38
After all, a refined Shmooper never lets a little hard work scare them off. [Boy smashes laptop]
- 00:41
So this question requires us to use Hess’s Law, which says that the total enthalpy change
- 00:46
of a chemical reaction is equal to the sum of enthalpy changes of each of the steps in
- 00:50
the reaction.
- 00:52
We also need to recognize that the first reaction given in the problem statement is the combustion [Propene combustion equation]
- 00:56
of propene.
- 00:57
So we want to find the enthalpy of this reaction.
- 01:01
If we can figure out how to add up some combination of the three reactions given in the problem
- 01:06
statement to make the overall combustion reaction, we can add up the enthalpies in the same way
- 01:11
to find the total reaction enthalpy.
- 01:13
No problem, right?
- 01:15
So let’s label these reactions I, II, and III and start adding them up to try to make
- 01:20
the overall combustion reaction. [Three reactions added together]
- 01:22
And remember, we have to follow Hess’s Law.
- 01:24
If you violate Hess’s Law, you have to go to Hess’s prison, and believe us, you don’t [People inside a prison cell]
- 01:28
want to go to Hess’s prison… that’s a mistake we’ll never make again.
- 01:31
First, we’ll add three times reaction II.
- 01:35
Then, add three times reaction III.
- 01:37
Finally, we’ll subtract reaction I, which is the same as adding the reverse reaction.
- 01:43
When we add all this up, we see some terms appear on both sides of the equation, so we
- 01:46
can subtract 3 H2 and 3 C from both sides.
- 01:50
Once we’ve done this, we’ve got the overall propene combustion reaction.
- 01:54
Now comes the most important part of Hess’s Law. [Woman teacher discussing Hess's Law]
- 01:57
We have to add up the enthalpies of reactions I, II, and III in exactly the same way as
- 02:01
we added up the reactions.
- 02:03
So if we take three times reaction II plus three times reaction III minus reaction I,
- 02:07
plug in the values, and do the math, we’ve got our answer:
- 02:12
-2093.3 kJ
- 02:14
If we round to the nearest ten, we can see that this is answer choice (D). [Answer D circled green]
- 02:18
Looky there, we did it, and without landing in Hess's Jail.
- 02:21
Hey, it's no laughing matter.
- 02:22
Last week our neighbor called Hess’s cops to come bust up a chemistry study group. [Study group eating chips together and cops appear]
- 02:27
We hear they're serving twenty to Avogadro's number.
Related Videos
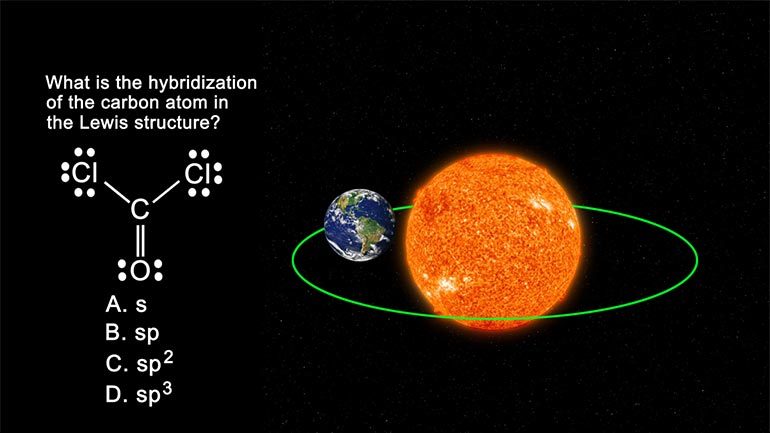
AP Chemistry: Structure of Atoms Drill 1, Problem 5. What is the hybridization of the carbon atom in the Lewis structure?
AP Chemistry DBQ/Free Response. Perform the following calculations.
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?