ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
AP Chemistry Videos 45 videos
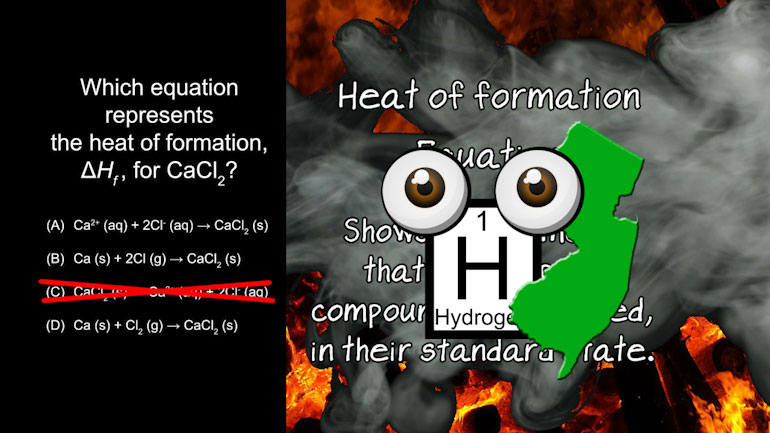
AP Chemistry 3.5 Chemical Reaction Rates. How much 85Kr will we need to collect?
Want to pull an Oliver Twist and ask us for more? We've gotcha covered. Head over to /video/subjects/test-prep/ap/ap-chemistry/ for more AP Ch...
AP Chemistry 1.3 Forming and Breaking Bonds. Which of the following ions are spectator ions?
AP Chemistry 2.3 Chemical Reaction Rates 3 Views
Share It!
Description:
AP Chemistry 2.3 Chemical Reaction Rates. What is the experimental rate law for this reaction?
Transcript
- 00:03
And here’s your Shmoop du jour, brought to you by reaction rate.
- 00:07
Hey, think fast! …Ooh, that’s gotta hurt. [Boy strikes a ball with a bat and ball hits man in face]
- 00:10
Sorry…
- 00:11
Here’s today’s problem:
- 00:12
The reaction: A + B yields C was run three different times, and each time, the concentrations
Full Transcript
- 00:18
of the reactants were altered.
- 00:20
The reaction rate was also measured each time the reaction was run, as shown in the table.
- 00:25
What is the experimental rate law for this reaction? [Table of experiment measurements]
- 00:28
And here are your potential answers.
- 00:31
To answer this question, we’ll need to examine the experimental data and deduce the appropriate
- 00:38
rate law for this reaction. [Doctor with a zombie-looking man on a table]
- 00:41
The reactants here are species A and B. In the three experiments shown in the table,
- 00:46
the initial concentrations of A and B are varied.
- 00:49
Because everyone is special in their own way. [Woman hugging a young boy]
- 00:51
Anyway, between experiments 1 and 3, the initial concentration of A is doubled while the initial
- 00:58
concentration of B stays the same.
- 01:00
We can use these two data points to find out how the rate depends on the concentration [Rate swinging and A grabs it]
- 01:04
of species A without worrying about species B.
- 01:06
So, what is the dependence on A? [A and B sitting on a couch]
- 01:09
When the initial concentration of A is doubled, the rate stays the same, so it doesn’t depend
- 01:14
on A at all.
- 01:15
Kind of like how your parents don’t depend on you to do the dishes anymore. [Girl sitting on a couch with a pizza]
- 01:18
Yeah, we know, you'd get to them "in a minute".
- 01:20
Whatever you say.
- 01:21
So between experiments 1 and 2, the initial concentration of B is doubled, while the initial
- 01:26
concentration of A stays the same.
- 01:28
With these two points, we can find how the rate depends on species B.
- 01:31
When the concentration of B is doubled, the rate increases by four times. [B increases in size and a hand holding 4 fingers appears]
- 01:36
That means the rate depends on B squared.
- 01:38
That's not quite like us… when our concentration is doubled, we make it through class without
- 01:42
falling asleep.
- 01:43
We know.
- 01:44
Not all heroes wear capes. [People staying awake in a classroom]
- 01:45
Anyway, now we know that the rate depends on the concentration of B squared, but it
- 01:50
doesn’t depend on A at all.
- 01:51
Looking at our answers, choice (A) fits the bill, as all the others include some dependence
- 01:56
on species A. So choice (A) is the right answer.
- 01:59
Now it’s time to get those dishes done. [A collection of dirty dishes]
- 02:03
Hey, where are you going?
- 02:05
Oh, brother.
- 02:06
Seriously, maybe your brother will do them instead. [Brother and sister run away]
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
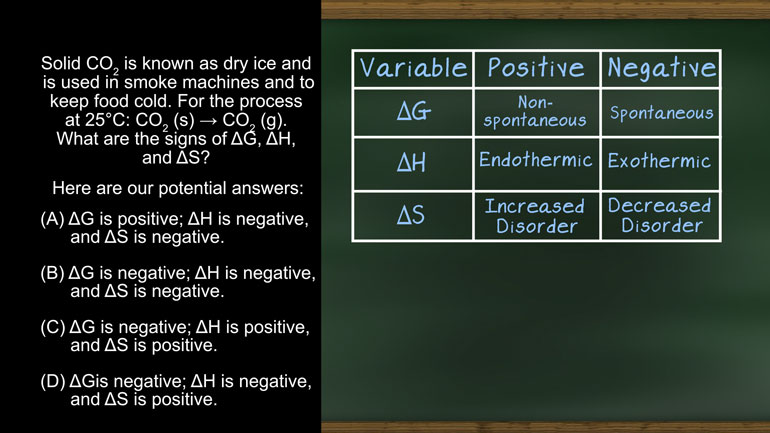
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?